Allegro
Screening Tympanometer
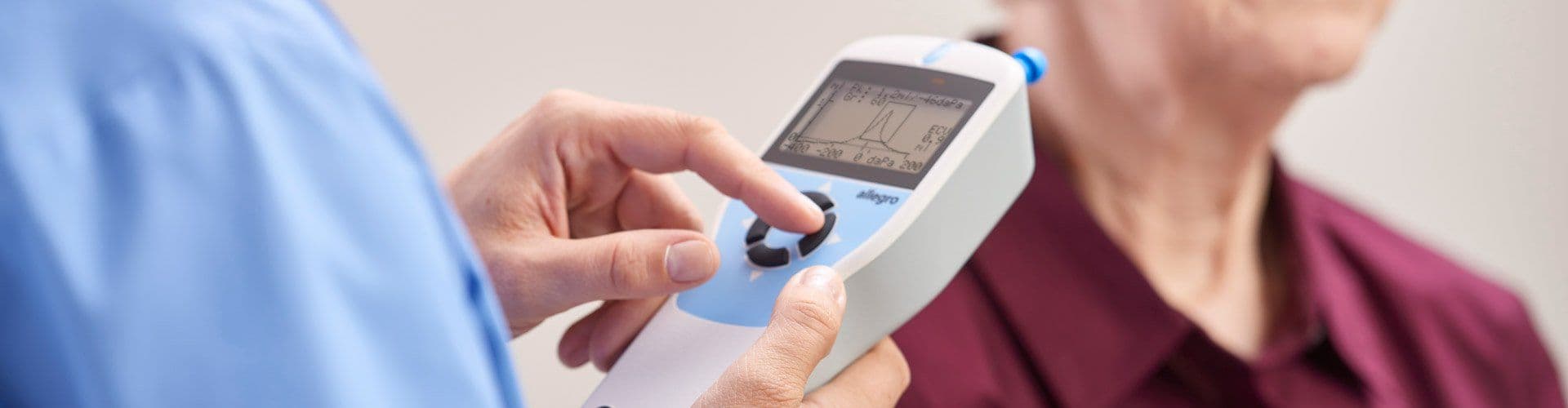
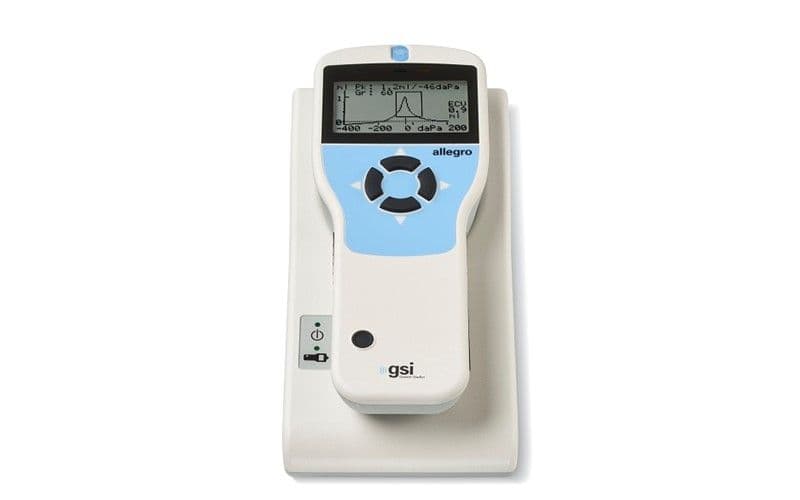
The GSI Allegro™ is a handheld screening tympanometer ready for any testing environment that requires tympanometry and ipsilateral reflexes. This middle ear analyzer offers a four button navigation that allows for quick and reliable tympanometry and acoustic reflex measurements. Automatic measurements of the middle ear status are completed in seconds using the device’s configurable test settings. The Allegro includes a charging cradle, thermal printer, and carrying case.
Request Quote
Frequently Asked Questions
What tests can be performed with the GSI Allegro?
The Allegro conducts middle ear screenings on a variety of patients. The device can perform tympanometry using a 226 Hz probe tone and ipsilateral acoustic reflex testing at 500, 1000, 2000, and 4000 Hz from 70 - 100 dB HL.
What middle ear measurements are displayed?
The Allegro displays four measurements. Automatic tympanic admittance peak, peak pressure, ear canal volume, and gradient are included.
What is the ear seal check?
The ear seal check ensures that adequate pressure can be created in the ear canal prior to performing middle ear testing. If a seal is obtained, the tympanogram will automatically be measured.
What settings can be customized on the Allegro?
Allegro makes it easy to customize settings for each patient. There are 17 settings the user can easily change. For example, the reflex frequencies, intensity step size, and intensity levels may be specified by the user.
Do I have to clean the probe tip on the Allegro?
Yes, cleaning the tip is necessary. The probe tip should be inspected every time prior to placing an ear tip. The small holes through the probe tip must be kept clear. If debris or blockage is visible, the probe tip must be removed from the unit and cleaned using a floss cleaning kit. It may be necessary to replace the probe tip if damage is visible.
Auto Start
Screenings start quickly with the Allegro. A tympanogram automatically begins recording as soon as the ear canal seal is obtained. No additional button presses on the tympanometer are needed. Therefore, testing can be completed quickly with challenging patients.
Product Specifications
• W x D x H: 4.5 in x 9 in x 2.8 in (11.5 cm x 23 cm x 7 cm)
• Display: 128 x 64 px / 8 lines of 21 characters
• Weight: 1.433 lb (650 g)
• Instrument Type: Meatus compensated tympanometer
• Analysis Performed: Admittance peak level (in ml); Peak pressure of same; Gradient (in daPa); Ear canal volume (ECV) at 200 daPa
• Probe Tone Levels and Accuracy: 226 Hz +/-2%; 85 dB SPL +/-2 dB over range 0.2 ml to 5 ml
• Pressure Levels and Accuracy: +200 daPa to -400 daPa +/-10 daPa or +/-10% (whichever is larger) over range
• Ear Volume Measurement Range and Accuracy: 0.2 ml to 5 ml +/-0.1 ml or +/-5% (whichever is larger) over entire range
• Sweep Speed: Typically 200 daPa/sec; dependent on ear/cavity volume
• Pressure Limits (Safety Cut Out): +600 to -800 daPa
• Measurement Modes: Ipsilateral
• Reflex Tone Levels and Accuracy: 500 Hz, 1 kHz, 2 kHz, 4 kHz (+/-2%); Configurable over range 70 dB to 100 dBHL (4 kHz restricted to 95 dBHL) +/-3 dB, referenced to 2 ml calibration volume; Compensates for measured ear volume
• Reflex Detection Threshold and Accuracy: 0.01 ml to 0.5 ml +/-0.01 ml configurable in 0.01 ml steps
• Reflex Analysis: Reflex present/absent at each level tested; maximum amplitude of each reflex (seen on printed report & computer report); pressure at which reflex was performed
• Pressure Used for Reflex Measurement: Pressure at Tympanogram peak, or 0 daPa
• Reflex Tone Duration: 0.6 seconds
• Number of Records Stored in Patient Database: 32 patients
• Data Stored: Patient initials, tympanogram, reflex graphs, analysis, time/date, and test parameters
• English
• German
• French
• Spanish
• Portuguese
• Italian
• Supported Printer: Sanibel MPT-II
• Interface: Wired connection to cradle
• USB Version 1.1
• Operating Temperature Range: +59° F (+15° C) to +95° F (+35° C)
• Operating Humidity Range: 30% to 90% RH, non-condensing
• Operating Atmospheric Pressure Range: 980 to 1040 mb, non-condensing
• Transport and Storage Temperature Range: -68° F (-20° C) to +158° F (+70° C)
• Transport and Storage Humidity Range: 10% to 90% RH, non-condensing
• Transport and Storage Atmospheric Pressure Range: 900 to 1100 mb
• Battery: NiMH rechargeable battery pack
• Interface: Wired connection to cradle
• Main Power (To Cradle): 100 - 240 Vac; 50/60 Hz; 0.2 A
• Number of Recordings with Full Charge: Up to 100
• Auto Power-off Delay: 90 or 180 seconds
• Idle Current: 70 mA
• Current While Testing: 230 mA
Manufactured, designed, developed and marketed under ISO 13485 certified quality systems
• IEC 60601-1 (plus UL, CSA & EN deviations)
• IEC 60601-1-2
• IEC 60645-5, Type 2 Tympanometer
• CE Mark: To the EU Medical Device Directive
As Fast as Three Seconds
Perform a tympanogram on a single ear in as little as three seconds. Quick hearing testing is crucial for testing a diverse patient population.
Training is Easy
New staff can begin testing on the portable tympanometer with confidence within a few minutes. The Allegro is a device that is easy to use and easy to train users, with its four button navigation and logical interface.
Lightweight Design
The Allegro is versatile and easy to transport. It is ideal for situations such as a satellite clinic or health fair, where a lightweight device that is portable is important. The tympanometer also comes with a convenient carrying case.
Signature Navigation
The Allegro provides a simple four button navigation pad, which is a signature feature on many GSI products. With just a few button presses, you can conduct testing for both tympanometry and acoustic reflex threshold measurements, as well as review and print results. It’s intuitive and simple for users to learn.
Key Tympanometer Features
1. Quick Seal
Quickly obtain a seal for tympanometry. Once a seal is obtained, the handheld tympanometer begins testing automatically.
2. Four Key Tymp Measurements
Four key tymp measurements include peak pressure, ear canal volume, tympanometric width, and admittance at peak.
3. Customizable User Settings
Global settings such as test sequence and test prompts may be defined by the user according to patient population or clinic needs.
4. Portable Design
The Allegro is designed with portability in mind. The device is lightweight and perfect for any testing environment, such as satellite clinics, inpatient rooms, health fairs, and general practice physician offices.
5. Simple Navigation
Four button navigation simplifies the testing procedures for a fast paced testing environment.
6. Patient Memory
Test multiple patients and manage the data at your convenience. The Allegro can store information from up to 32 patient test results to be analyzed and managed at a different time.
Featured Videos
PRODUCT TUTORIAL
Basic Device Operation
PRODUCT TUTORIAL
What Happens During Testing
PRODUCT TUTORIAL
Reporting Options
New Tutorial Video
Follow the link to access the new Allegro tutorial, along with other training materials. Topics covered include:
- Basic Device Operation
- Preparing the Patient
- What Happens During Testing
- Reporting Options
Easy Charging, Convenient Printing
The Allegro comes with a portable charging cradle so you’re never without a functioning tympanometer. A fully charged device will last approximately eight hours for testing. Seamlessly print from the cradle when the device is connected to the charging cradle.
Visit the Tympanometers Page for a full list of available GSI tympanometers.
Next Steps
Learn more by checking out our materials below or clicking the distributor locator button.